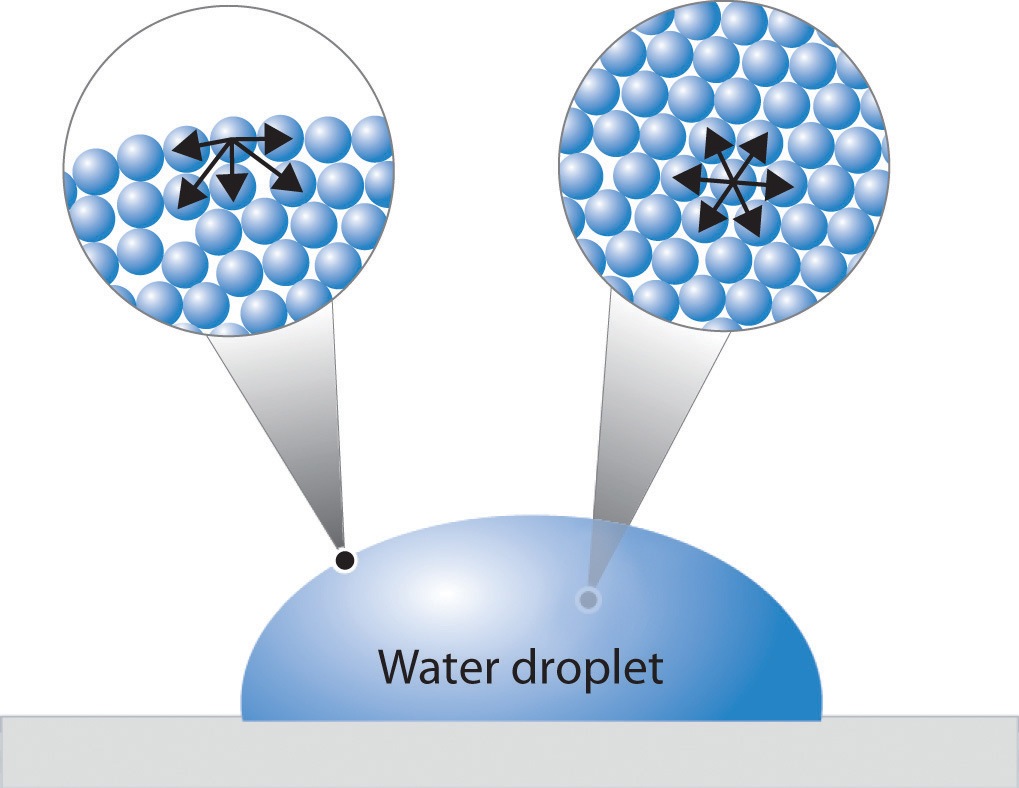
How Does Surface Tension Relate To Water . This phenomenon can be observed in the nearly. (b) an iron needle similarly dents a water surface until the restoring force (surface tension) grows to equal its weight. Surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water (figure \(\pageindex{2}\) ). An example of such an. The shape of water and mercury meniscuses in a test tube depends on cohesive and adhesive forces. Unravel the enigma of surface tension and adhesion in liquids, particularly water. The cohesive forces between liquid. Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. Water has a high surface tension because the water molecules on the surface are pulled together. Water has a surface tension of 72 mn/m and can easily wet surfaces.
from chem.libretexts.org
Water has a surface tension of 72 mn/m and can easily wet surfaces. The shape of water and mercury meniscuses in a test tube depends on cohesive and adhesive forces. This phenomenon can be observed in the nearly. An example of such an. Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. Unravel the enigma of surface tension and adhesion in liquids, particularly water. The cohesive forces between liquid. Water has a high surface tension because the water molecules on the surface are pulled together. Surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water (figure \(\pageindex{2}\) ). (b) an iron needle similarly dents a water surface until the restoring force (surface tension) grows to equal its weight.
Surface Tension Chemistry LibreTexts
How Does Surface Tension Relate To Water Unravel the enigma of surface tension and adhesion in liquids, particularly water. Surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water (figure \(\pageindex{2}\) ). The shape of water and mercury meniscuses in a test tube depends on cohesive and adhesive forces. Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. Water has a surface tension of 72 mn/m and can easily wet surfaces. Water has a high surface tension because the water molecules on the surface are pulled together. Unravel the enigma of surface tension and adhesion in liquids, particularly water. This phenomenon can be observed in the nearly. (b) an iron needle similarly dents a water surface until the restoring force (surface tension) grows to equal its weight. An example of such an. The cohesive forces between liquid.
From www.biolinscientific.com
Surface tension of water Why is it so high? How Does Surface Tension Relate To Water An example of such an. Water has a high surface tension because the water molecules on the surface are pulled together. This phenomenon can be observed in the nearly. Unravel the enigma of surface tension and adhesion in liquids, particularly water. (b) an iron needle similarly dents a water surface until the restoring force (surface tension) grows to equal its. How Does Surface Tension Relate To Water.
From dornshuld.chemistry.msstate.edu
1.4 Surface Tension Chemistry How Does Surface Tension Relate To Water This phenomenon can be observed in the nearly. (b) an iron needle similarly dents a water surface until the restoring force (surface tension) grows to equal its weight. Unravel the enigma of surface tension and adhesion in liquids, particularly water. Water has a surface tension of 72 mn/m and can easily wet surfaces. The cohesive forces between liquid. Water has. How Does Surface Tension Relate To Water.
From www.slideserve.com
PPT Properties of Liquids PowerPoint Presentation, free download ID How Does Surface Tension Relate To Water Surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water (figure \(\pageindex{2}\) ). Water has a surface tension of 72 mn/m and can easily wet surfaces. Unravel the enigma of surface tension and adhesion in liquids, particularly water. The shape of water and mercury meniscuses in a test tube. How Does Surface Tension Relate To Water.
From civilchapola.com
What's Surface Tension in Fluid Mechanics Water Droplet & Soap Bubble How Does Surface Tension Relate To Water This phenomenon can be observed in the nearly. Surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water (figure \(\pageindex{2}\) ). Water has a high surface tension because the water molecules on the surface are pulled together. Water has a surface tension of 72 mn/m and can easily wet. How Does Surface Tension Relate To Water.
From www.myxxgirl.com
Mechanical Properties Of Fluids Class Surface Tension Based On My XXX How Does Surface Tension Relate To Water This phenomenon can be observed in the nearly. The shape of water and mercury meniscuses in a test tube depends on cohesive and adhesive forces. An example of such an. Water has a high surface tension because the water molecules on the surface are pulled together. (b) an iron needle similarly dents a water surface until the restoring force (surface. How Does Surface Tension Relate To Water.
From civilchapola.com
What's Surface Tension in Fluid Mechanics Water Droplet & Soap Bubble How Does Surface Tension Relate To Water (b) an iron needle similarly dents a water surface until the restoring force (surface tension) grows to equal its weight. Water has a high surface tension because the water molecules on the surface are pulled together. The cohesive forces between liquid. Unravel the enigma of surface tension and adhesion in liquids, particularly water. This phenomenon can be observed in the. How Does Surface Tension Relate To Water.
From recemev.jimdo.com
Surface Tension recemev How Does Surface Tension Relate To Water Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. Unravel the enigma of surface tension and adhesion in liquids, particularly water. This phenomenon can be observed in the nearly. Surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water. How Does Surface Tension Relate To Water.
From www.youtube.com
Surface Tension and Surfactant (Fluid Mechanics Lesson 12) YouTube How Does Surface Tension Relate To Water Unravel the enigma of surface tension and adhesion in liquids, particularly water. An example of such an. The cohesive forces between liquid. This phenomenon can be observed in the nearly. Water has a high surface tension because the water molecules on the surface are pulled together. The shape of water and mercury meniscuses in a test tube depends on cohesive. How Does Surface Tension Relate To Water.
From www.slideserve.com
PPT Chapter 3 Water and Life PowerPoint Presentation, free download How Does Surface Tension Relate To Water Unravel the enigma of surface tension and adhesion in liquids, particularly water. The cohesive forces between liquid. Surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water (figure \(\pageindex{2}\) ). An example of such an. The shape of water and mercury meniscuses in a test tube depends on cohesive. How Does Surface Tension Relate To Water.
From www.sciencefacts.net
Surface Tension Definition, Examples, and Unit How Does Surface Tension Relate To Water The cohesive forces between liquid. Surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water (figure \(\pageindex{2}\) ). The shape of water and mercury meniscuses in a test tube depends on cohesive and adhesive forces. Unravel the enigma of surface tension and adhesion in liquids, particularly water. (b) an. How Does Surface Tension Relate To Water.
From www.australianenvironmentaleducation.com.au
Water is found everywhere on earth, so why is it important? How Does Surface Tension Relate To Water An example of such an. (b) an iron needle similarly dents a water surface until the restoring force (surface tension) grows to equal its weight. Water has a high surface tension because the water molecules on the surface are pulled together. Unravel the enigma of surface tension and adhesion in liquids, particularly water. Surface tension, property of a liquid surface. How Does Surface Tension Relate To Water.
From www.slideserve.com
PPT Lesson 3 Cohesion, adhesion & surface tension PowerPoint How Does Surface Tension Relate To Water Water has a high surface tension because the water molecules on the surface are pulled together. Unravel the enigma of surface tension and adhesion in liquids, particularly water. Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. The cohesive forces between liquid. Water has a surface tension of 72 mn/m. How Does Surface Tension Relate To Water.
From www.docdroid.net
Application_of_surface_tension_in_fluid_flow_1651146905.pdf DocDroid How Does Surface Tension Relate To Water Water has a surface tension of 72 mn/m and can easily wet surfaces. (b) an iron needle similarly dents a water surface until the restoring force (surface tension) grows to equal its weight. Water has a high surface tension because the water molecules on the surface are pulled together. The cohesive forces between liquid. Unravel the enigma of surface tension. How Does Surface Tension Relate To Water.
From www.slideshare.net
Measuring the Surface Tension of Water by Light Diffraction on Capill… How Does Surface Tension Relate To Water (b) an iron needle similarly dents a water surface until the restoring force (surface tension) grows to equal its weight. Water has a high surface tension because the water molecules on the surface are pulled together. This phenomenon can be observed in the nearly. Water has a surface tension of 72 mn/m and can easily wet surfaces. An example of. How Does Surface Tension Relate To Water.
From guidemanualspyglasses.z14.web.core.windows.net
How To Explain Surface Tension How Does Surface Tension Relate To Water An example of such an. Unravel the enigma of surface tension and adhesion in liquids, particularly water. Surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water (figure \(\pageindex{2}\) ). This phenomenon can be observed in the nearly. The shape of water and mercury meniscuses in a test tube. How Does Surface Tension Relate To Water.
From cynthianalibrary.org
Surface Tension Sink or Float? How Does Surface Tension Relate To Water Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. (b) an iron needle similarly dents a water surface until the restoring force (surface tension) grows to equal its weight. This phenomenon can be observed in the nearly. Surface tension of water can cause things to float which are denser than. How Does Surface Tension Relate To Water.
From www.thoughtco.com
Surface Tension Definition in Chemistry How Does Surface Tension Relate To Water Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. (b) an iron needle similarly dents a water surface until the restoring force (surface tension) grows to equal its weight. Surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water. How Does Surface Tension Relate To Water.
From www.slideserve.com
PPT HOW SOAP WORKS PowerPoint Presentation, free download ID1114540 How Does Surface Tension Relate To Water Water has a high surface tension because the water molecules on the surface are pulled together. The cohesive forces between liquid. This phenomenon can be observed in the nearly. (b) an iron needle similarly dents a water surface until the restoring force (surface tension) grows to equal its weight. The shape of water and mercury meniscuses in a test tube. How Does Surface Tension Relate To Water.